Determine the equilibrium constant, Keq, at 25°C for the reaction 2Br- (aq) + I2(s) 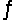
Br2(l) + 2I- (aq)
Definitions:
Total Dollar Return
The total gain or loss on an investment over a specific period, combining both capital gains and any dividends or interest received.
Dividends
Payments made by a corporation to its shareholders, usually derived from the company's profits.
SML
Stands for the Security Market Line, which represents the expected return of a market portfolio as a function of systematic, or market, risk.
Risk-Free Rate
The theoretical rate of return of an investment with no risk of financial loss.
Q4: The molar solubility of manganese(II)carbonate is 4.2
Q11: An essential amino acid is one that<br>A)must
Q15: Write the chemical formula of dolomite that
Q16: Write the chemical formula of pyrite.
Q26: How many grams of copper are deposited
Q47: Determine the equilibrium constant, K<sub>eq</sub>, at
Q49: Calculate the pH at the equivalence point
Q65: The compound CH<sub>3</sub>NH<sub>2</sub> reacts with water to
Q73: Complete and balance the following redox
Q91: Assuming <span class="ql-formula" data-value="\Delta"><span class="katex"><span