Given the following standard reduction potentials in acid solution 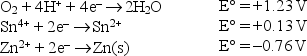 write the formula of the strongest reducing agent.
write the formula of the strongest reducing agent.
Definitions:
Management Problems
Issues or challenges faced by the management of an organization, which can include employee performance, resource allocation, and executing strategic plans.
Open System
A concept where systems interact with their environment through the exchange of information, energy, or material, which influences their structure and function.
Input
Resources, data, or efforts put into a system or process in order to achieve outcomes or results.
Special Type
A unique or distinct category, characterized by specific attributes that set it apart from others.
Q7: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q20: Using the thermodynamic data provided below, calculate
Q22: Write chemical equations that show what happens
Q24: Will a precipitate of magnesium fluoride form
Q27: Which one of the following reagents is
Q71: Solid sodium iodide is slowly added to
Q93: Which is an exception to the rule:
Q99: Which of the following is correct with
Q104: The equilibrium constant at 427°C for
Q125: Which one of the following statements is