For the reaction 3Fe(s) + C(s) 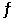 Fe3C(s) , H° = 21 kJ/mol and S° = 20.4 J/mol·K at 25°C. Estimate the minimum temperature above which the formation of cementite (Fe3C) is favored.
Fe3C(s) , H° = 21 kJ/mol and S° = 20.4 J/mol·K at 25°C. Estimate the minimum temperature above which the formation of cementite (Fe3C) is favored.
Definitions:
Post-Structural Theory
An intellectual movement in criticism and philosophy that suggests structures and systems of thought or language are inherently unstable and subject to deconstruction.
Knowledge Production
The process through which new information, understandings, and insights are generated, often through research and analysis in academic and scientific contexts.
High School
An educational institution that provides secondary education, typically for students aged approximately 14 to 18, preceding college or university.
Symbolic Interactionist
A sociological perspective focusing on how individuals interpret and give meaning to symbols, interactions, and social relationships in their daily lives.
Q7: How should a revaluation entry generally not
Q12: What is "fair value less costs to
Q30: One of the steps in the
Q52: The solubility of strontium carbonate is 0.0011
Q64: What is the coordination number of chromium
Q70: Calculate the pOH of a solution containing
Q100: Complete and balance the following redox
Q106: How many minutes would be required
Q112: Consider the following standard reduction potentials in
Q116: On December 31,2018,CA Inc.had a machine with