Estimate the age of a bottled wine that has a tritium, 3H, content 60% that of freshly bottled wine.Tritium decays by beta decay and has a half-life of 12.3 yr. 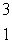 H
H 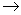
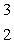 He +
He + 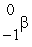
Definitions:
Nonpolar Covalent
A type of chemical bond where two atoms share a pair of electrons equally, typically between atoms of similar electronegativity.
Negatively Charged
describes an object or particle that has more electrons than protons, resulting in a net negative electric charge.
Synthesis Reaction
A chemical reaction in which two or more substances combine to form a more complex product.
Decomposition Reaction
A chemical reaction in which a single compound breaks down into two or more simpler substances.
Q11: Which one of these elements is not
Q14: Alkenes have the general formula<br>A)C<sub>n</sub>H<sub>2n-4</sub><br>B)C<sub>n</sub>H<sub>2n-2</sub><br>C)C<sub>n</sub>H<sub>2n</sub><br>D)C<sub>n</sub>H<sub>2n+2</sub><br>E)C<sub>n</sub>H<sub>2n+4</sub>
Q36: Which of these species is not an
Q37: At 700 K, the equilibrium constant
Q52: Which functional group, when present in a
Q54: In the coordination compound [Pt(NH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub>], the coordination
Q54: When an aqueous solution of NaCl is
Q92: Complete and balance the following redox
Q93: Complete and balance the nuclear equation <img
Q98: Balance the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg" alt="Balance the