At 700 K, the equilibrium constant for the reaction CO(g)+ H2O(g) 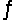 CO2(g)+ H2(g)is 5.10.What is G° for this reaction at this temperature?
CO2(g)+ H2(g)is 5.10.What is G° for this reaction at this temperature?
Definitions:
Captive Finance Company
A financial institution created by a company to provide loans and financial services exclusively to its customers.
Credit Extension
The act of providing a loan or increasing the credit limit available to a borrower, often by a financial institution.
Q2: Draw and label the geometric isomers of
Q35: Calculate the standard cell emf for the
Q39: Which one of the following statements
Q94: Ethanol and acetic acid react to form
Q95: Consider the chemical reaction 2NH<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q102: Given the following standard reduction potentials,
Q110: Which of these species has the highest
Q110: Charcoal samples taken from holes dug at
Q122: Aluminum metal is formed by the
Q124: Consider the reaction Fe + Sn<sup>2+</sup>(1