The boiling point of 1,4-butanediol is 230°C. Would you expect this compound to be soluble or insoluble in room-temperature water? 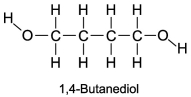
Definitions:
Boiling Point
The temperature at which a liquid's vapor pressure equals the external pressure, causing it to transition to gas.
Butan-1-ol
A four-carbon chain alcohol with the hydroxyl group attached to the first carbon.
1-butanamine
A chemical compound, also known as butylamine, which is an amine with the formula CH3(CH2)3NH2, used in the manufacture of pharmaceuticals and pesticides.
IUPAC Name
The systematic name given to a chemical substance, based on a set of standard rules by the International Union of Pure and Applied Chemistry.
Q15: How does the purchase and use of
Q46: Water, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2711/.jpg" alt="Water, O,
Q67: In the laboratory, endothermic reactions are usually
Q76: What coefficients balance the following equation? _
Q96: The hydroxide ion, HO<sup>-</sup>, is a _.<br>A)unique
Q102: What is the main difference between a
Q115: What is a half-life?<br>A)It is the time
Q120: Steel wool wetted with vinegar is sealed
Q130: Why is it important for a chemist
Q146: If an atom were the size of