How many moles of water, 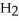 O, are produced from the reaction of 16 grams methane, C
O, are produced from the reaction of 16 grams methane, C 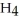 , with an unlimited supply of oxygen,
, with an unlimited supply of oxygen, 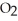 . How many grams of
. How many grams of 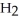 O is this? C
O is this? C 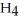 + 2
+ 2 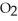 → C
→ C 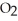 + 2
+ 2 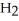 O
O
Definitions:
Salespeople
Individuals who are responsible for selling products or services, often working on commission and representing a company in dealings with customers.
Pearson Correlation
A measure of the linear correlation between two variables, giving a value between -1 and 1 where 1 implies a perfect positive linear relationship, -1 implies a perfect negative linear relationship, and 0 implies no linear correlation.
Regression Line
A linear representation that best fits the distribution of data points in scatter plotting, predicting the relationship between dependent and independent variables.
Coefficient Of Determination
A statistical measure, often denoted as R^2, that represents the proportion of the variance in the dependent variable predictable from the independent variable(s) in a regression analysis.
Q19: An adhesive force is best described as
Q78: Steel wool wetted with vinegar is stuffed
Q81: Why is 2,4,5-trifluorophenol much more acidic than
Q82: A walnut stuck to a pin is
Q92: The following set of redox reactions takes
Q100: Qualitatively, what happens to the hydroxide ion
Q122: How many grams of sodium chloride are
Q134: Which of the following molecules would you
Q142: Describe what usually happens to a hot
Q153: Classify the following bonds as ionic, covalent,