Which should be a stronger base: ammonia, 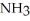 , or trifluoronitrogen,
, or trifluoronitrogen, 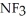 ? Why?
? Why? 
Definitions:
Pseudonym
A fictitious name used by an author instead of their real name, often to conceal identity or adopt a different persona in their writings.
Social Activism
The act of advocating for, intervening in, or participating in efforts to promote social, political, economic, or environmental reform or stability.
Conflict Theorists
Scholars who apply and develop the conflict theory perspective, focusing on how power disparities and social inequality drive social dynamics and change.
Social Facts
Concepts in sociology introduced by Émile Durkheim referring to societal norms, values, and structures that exist outside the individual but exert a coercive influence on behavior.
Q11: How many electrons are gained or lost
Q18: Fats and carbohydrates have similar chemical composition.
Q35: Alkaloid salts are not very soluble in
Q52: Another name for the "alcohol" of alcoholic
Q53: Which of the following might have the
Q59: The warming of a saturated solution of
Q67: Why is the formation of iron hydroxide,
Q79: Hard water contains excessive amounts of _.<br>A)fluoride
Q85: Which of the following statements about strong
Q90: The reactants shown schematically below represent iron