Why is the formation of iron hydroxide, Fe 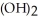 , from
, from 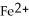 and O
and O 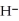 not considered an oxidation-reduction reaction?
not considered an oxidation-reduction reaction?
Definitions:
Welland Canal
A ship canal in Canada, connecting Lake Ontario and Lake Erie, facilitating navigation and trade by bypassing Niagara Falls.
Ballast Water
Water carried in ships' ballast tanks to improve stability and balance, which, when discharged, can introduce invasive aquatic species to new environments.
Autonomy
The degree to which a job provides the employee with the freedom, independence, and discretion to schedule their work and determine how it is done.
Responsibility
The state or fact of having a duty to deal with something or of having control over someone.
Q28: Camphor is a 10-carbon odoriferous natural product
Q29: Which number set identifies the longest chain
Q43: What is the number of grams of
Q53: What element is oxidized in the following
Q60: Which of the following would be an
Q90: The reactants shown schematically below represent iron
Q112: Peanut butter has more protein per gram
Q130: What is unique about the polysaccharides that
Q138: A common source of DNA damage is
Q145: Which of the following molecules could be