The amphoteric reaction between two water molecules is endothermic, which means that this reaction requires the input of energy in order to proceed: Energy + 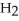 O +
O + 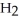 O →
O → 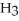
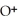 + O
+ O 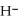 The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of
The warmer the temperature of the water, the more thermal energy is available for this reaction, and the more hydronium and hydroxide ions are formed. Based on this information, should the value of 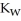 be expected to increase, decrease, or stay the same with increasing temperature?
be expected to increase, decrease, or stay the same with increasing temperature?
Definitions:
EBIT
This metric, known as Earnings Before Interest and Taxes, calculates a business's profitability without taking into account expenses from interest and taxes.
ROE
Return On Equity represents a financial performance metric that is obtained by dividing a company's net income by its shareholders' equity, demonstrating the company's effectiveness in using investor funds to grow earnings.
EPS
Earnings Per Share, a key indicator of a company's profitability, calculated by dividing the company's net income by its total number of outstanding shares.
DFL
Degree of Financial Leverage, a ratio measuring the sensitivity of a company's earnings per share to fluctuations in its operating income, due to changes in its capital structure.
Q3: What is the purpose of adding aluminum
Q22: How does an atom's electronegativity relate to
Q26: Why does oxygen have such a low
Q47: As an ice cube floating in a
Q65: What is the molarity of 0.50 liters
Q79: How do fats compare to other biomolecules
Q81: Why is 2,4,5-trifluorophenol much more acidic than
Q88: What is the cause of lactose intolerance?<br>A)You
Q91: Which of the following molecules contains a
Q92: Which of the following statements about polysaccharides