When a hydronium ion concentration equals 1 × 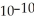 moles per liter, what is the pH of the solution? Is the solution acidic or basic?
moles per liter, what is the pH of the solution? Is the solution acidic or basic?
Definitions:
Nonemotional Information
Data or facts that do not evoke feelings or emotional responses, typically objective or neutral in nature.
Historical Events
Significant occurrences in history that have had a profound effect on societies, cultures, or the course of human development.
Personal Experiences
Events or occurrences that an individual personally goes through, shaping their perception and understanding of the world.
General Aggression Model
A comprehensive framework that explains the complex process by which individual, situational, and sociocultural factors contribute to aggressive behavior.
Q18: What is the boiling temperature of a
Q20: Which of the following statements describes an
Q22: Which of the following is not a
Q54: When a hydronium ion concentration equals 1
Q70: The air that a scuba diver breathes
Q109: The formation of hydrogen bonds between water
Q113: Given the following specific heats and that
Q120: What happens to the pH of a
Q137: Which of the following molecules contains a
Q138: Ice is slippery because _.<br>A)air molecules have