Hydrogen sulfide, H2S, burns in the presence of oxygen, 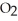 , to produce water,
, to produce water, 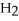 O, and sulfur dioxide, SO2. Through this reaction, is sulfur oxidized or reduced? 2
O, and sulfur dioxide, SO2. Through this reaction, is sulfur oxidized or reduced? 2 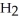 S + 3
S + 3 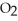 → 2
→ 2 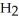 O + 2
O + 2 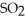
Definitions:
Financial Position
Represents the net worth of an entity, detailing assets, liabilities, and shareholders' equity at a specific point in time.
Assignable Loan Contract
A loan agreement that allows the lender to transfer or assign the loan to another party.
Finance Company
A financial institution that offers loans, leases, and other financial services to consumers and businesses.
Conditional Sales Contract
An agreement for the purchase of goods that does not fully transfer ownership to the buyer until certain conditions, typically full payment, have been met.
Q38: Why do most steroids have chemical structures
Q62: What is the leading cause of surface
Q82: A walnut stuck to a pin is
Q100: Which region (a-b-c)is an alcohol functional group?<br>A)a<br>B)b<br>C)c<br>D)b
Q103: Given the following specific heats and that
Q109: The formation of hydrogen bonds between water
Q117: Why does water not freeze at 0°C
Q143: How many structural isomers are shown here?
Q145: Which of the following molecules could be
Q153: The compound 6-aminohexanoic acid is used to