For each of the following unbalanced equations, which is depicted: oxidation or reduction? a) Cr → 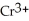 b) Sn →
b) Sn → 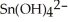
Definitions:
College Education
A post-secondary education offered by colleges and universities, leading to an associate's, bachelor's, master's, or doctoral degree.
Two-Parent Homes
Households where children live with both of their biological or adoptive parents.
Blu-Ray Players
Devices designed to play Blu-ray discs, which offer high-definition video and data storage greater than that of traditional DVDs.
Store A
A placeholder name often used to reference a specific retail location or business entity in discussions or case studies.
Q1: Iron atoms, Fe, are better reducing agents
Q31: For the following balanced reaction, which of
Q39: The following statement describes what level of
Q56: What is the difference between a beta
Q89: Which of the following is the hallmark
Q99: How many electrons are transferred from iron
Q117: How are proteins similar to starch?<br>A)Both are
Q118: Because vitamins are so good for us,
Q127: What would the concentration of H<sub>3</sub>O<sup>+</sup> be
Q154: Which of the following does not contain