Iron atoms, Fe, are better reducing agents than copper ions, 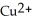 . In which direction do electrons flow when an iron nail is submerged in a solution of
. In which direction do electrons flow when an iron nail is submerged in a solution of 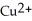 ions?
ions?
Definitions:
Intercalated Discs
Specialized structures in cardiac muscle cells that facilitate the synchronization of heart contraction through electrical and mechanical connections.
Clot Formation
The process by which blood changes from a liquid to a gel, forming a clot, which is essential in preventing excessive bleeding when blood vessels are injured.
Thrombin
An enzyme in blood plasma that causes the clotting of blood by converting fibrinogen to fibrin.
Prothrombin
A protein present in blood plasma that is converted into active thrombin during coagulation, playing a key role in blood clot formation.
Q15: In a battery, the following two oxidation-reduction
Q61: According to the figure above, which amino
Q68: For the following acid-base reaction, identify what
Q77: Is the synthesis of ozone, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2711/.jpg"
Q85: Consider the following molecules. What is the
Q98: Which of the following compounds is an
Q124: Which of the following does not describe
Q139: If it takes three golf balls to
Q141: Why is heat often added to chemical
Q142: Would you expect polypropylene to be denser