What element is oxidized in the following equation and what element is reduced? 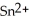 + 2 Ag → Sn + 2 Ag⁺
+ 2 Ag → Sn + 2 Ag⁺
Definitions:
Advertising Campaign
is a series of coordinated promotional efforts across various media channels aimed at achieving specific marketing objectives.
Group Development Process
The group development process includes stages through which a group progresses, from formation to performing, as it matures and works towards achieving its goals.
Group Development Process
A sequence of stages that a group goes through from formation to maturity, including forming, storming, norming, performing, and adjourning.
Cement
A binding material, often used in construction, made by grinding calcined limestone and clay to a fine powder which hardens when mixed with water.
Q7: When an unknown peptide containing five amino
Q7: What is the source of acid rain?<br>A)Acid
Q43: Which direction do the photo-ejected electrons in
Q53: What element is oxidized in the following
Q60: Which of the following metals would be
Q73: If it takes three carbon atoms to
Q78: How do opiates like morphine work?<br>A)They block
Q109: Ethanol can be made industrially from two
Q113: Given the following specific heats and that
Q113: If you were to compare the synthesis