Given the data in the table below, ΔH°rxn for the reaction 4NH3 (g) + 5O2 (g) → 4NO (g) + 6H2O (l)
Is ________ kJ. 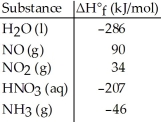
Definitions:
Higher Horsepower
Refers to engines or motors with greater capacity to produce power, typically resulting in enhanced performance or capability.
Right Price
The optimal price point for a product or service that balances profitability with customer satisfaction and demand.
Market Will Bear
The maximum price that consumers are willing to pay for a product or service in a given market.
Fixed Costs
Expenses that do not change with the level of goods or services a business produces, such as rent, salaries, and loan payments.
Q28: Write the balanced equation for the reaction
Q29: The wavelength of light that has a
Q31: The compound HClO<sub>4</sub> is a weak acid.
Q37: The mass % of C in methane
Q80: Consider the following properties of an element:
Q135: The spectator ions in the reaction between
Q136: Based on the octet rule, iodine most
Q137: Which hydroxides are strong bases? Sr(OH)<sub>2</sub><br>KOH<br>NaOH<br>Ba(OH)<sub>2</sub><br>A)KOH, Ba(OH)<sub>2</sub><br>B)KOH,
Q158: Which periodic table group contains only nonmetals?<br>A)8A<br>B)2A<br>C)6A<br>D)7A<br>E)5A
Q206: The element _ is the most similar