Given the data in the table below, ΔH°rxn for the reaction 4NH3 (g) + 5O2 (g) → 4NO (g) + 6H2O (l)
Is ________ kJ. 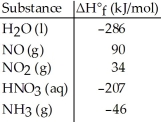
Definitions:
Quaker Belief
A set of principles held by members of the Religious Society of Friends, characterized by pacifism, simplicity, and a direct personal relationship with God.
Preach
To deliver a religious message or sermon, often involving moral or spiritual guidance and interpretation of religious texts.
Male or Female
Gender classification typically based on biological and physical differences at birth.
King Philip's War
A conflict between American Indian inhabitants of New England and English colonists and their Indian allies from 1675 to 1676.
Q4: The wavelength of radio waves can be
Q11: What volume (mL)of 7.48 × 10<sup>-2</sup> M
Q36: The primary component of natural gas is
Q46: The electron configuration of the atom with
Q75: When the following equation is balanced, the
Q83: How many grams of phenol (C<sub>6</sub>H<sub>5</sub>OH)are contained
Q90: The Lewis structure of the CO<sub>3</sub><sup>2-</sup> ion
Q119: The value of ΔH° for the reaction
Q170: Which two elements have the same ground-state
Q242: How many protons does the Br<sup>-</sup> ion