Given the data in the table below, ΔH°rxn for the reaction PCl3 (g) + 3 HCl (g) → 3Cl2 (g) + PH3 (g)
Is ________ kJ. 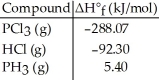
Definitions:
Equation
A mathematical statement that asserts the equality between two expressions, typically involving variables and constants.
Initial Velocity
Initial velocity is the velocity of an object at the start of its motion, often considered in the study of mechanics within physics.
Model Equation
A mathematical representation that shows the relationships between different variables.
Q59: Of the alkaline earth metals, which two
Q75: The reaction of a metal with a
Q78: The type of compound that is most
Q82: What is the enthalpy change (in kJ)of
Q88: Which one of the following is considered
Q109: Nonmetals can be _ at room temperature.<br>A)solid,
Q131: The value of ΔH° for the reaction
Q137: A valid Lewis structure of _ cannot
Q171: The balanced reaction between aqueous nitric acid
Q174: The formula weight of magnesium fluoride (MgF<sub>2</sub>),