Given the data in the table below, ΔH°rxn for the reaction Ag2O (s) + H2S (g) → Ag2S (s) + H2O (l)
Is __________ kJ. 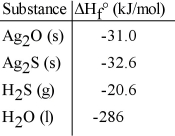
Definitions:
Unexplained Variation
The portion of the total variation in a data set that is not accounted for by the statistical model being used.
P-value
The chance of getting results that are as extreme or more extreme than what was actually seen, assuming the null hypothesis holds.
Average Salary
The mean amount of payment earned by employees in a certain period.
Multiple Linear Regression
A statistical technique that models the relationship between a dependent variable and two or more independent variables by fitting a linear equation to observed data.
Q10: The value of ΔH° for the reaction
Q35: Given the data in the table below,
Q69: A sample of CH<sub>2</sub>F<sub>2</sub> with a mass
Q88: Petroleum is a liquid composed of hundreds
Q103: There are _ oxygen atoms in 30
Q103: In the Lewis structure of ClF, the
Q110: What is the wavelength of light (nm)that
Q120: Of the following, which gives the correct
Q147: The value of ΔH° for the reaction
Q151: An atom of <sup>131</sup>Xe contains _ electrons.<br>A)131<br>B)185<br>C)77<br>D)123<br>E)54