Given the data in the table below, ΔH°rxn for the reaction 2 Ag2S (s) + O2 (g) → 2 Ag2O (s) + 2S (s)
Is __________ kJ. 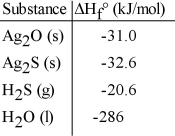
Definitions:
True Inequality
A statement of inequality that is valid for the values it involves.
Real Number Line
A line that graphically represents all possible real numbers, both positive and negative, including integers and fractions.
Ascending Order
Arranging numbers or other elements in a sequence from the smallest to the largest value.
Approximate
To approximate means to find a value close to the exact answer, often through estimation or rounding.
Q8: The type of compound that is most
Q21: The _ quantum number defines the shape
Q28: Using the Born-Haber cycle, the ΔH<sub>f</sub>° of
Q41: With which of the following will the
Q71: Gaseous argon has a density of 1.40
Q85: When the following equation is balanced, the
Q87: The name of the ionic compound RbBrO<sub>4</sub>
Q92: When the following equation is balanced, the
Q117: How many sulfur dioxide molecules are there
Q157: A 0.200 M K<sub>2</sub>SO<sub>4 </sub>solution is produced