At 27°C, Kp = 0.095 for the equilibrium: NH4HS (s) 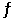 NH3 (g) + H2S (g)
NH3 (g) + H2S (g)
A sample of solid NH4HS is placed in a closed vessel and allowed to equilibrate. Calculate the equilibrium partial pressure (atm) of ammonia, assuming that some solid NH4HS remains.
Definitions:
AVC
Average Variable Cost, which represents the variable costs of production (costs that change with output) divided by the quantity of output.
Average Total Cost
Calculated as the sum of all production costs divided by the number of units produced, representing the per unit cost of production.
Average Variable Cost
The sum of all costs that vary with output levels, divided by the total output generated.
Economies of Scale
The cost advantage that arises with increased output of a product, as the fixed costs are spread over more units of production.
Q17: Classify the following compounds as weak acids
Q19: CsCl crystallizes in a unit cell that
Q40: What is the solubility (in M)of PbCl<sub>2</sub>
Q54: The fourth most abundant component of dry
Q57: What is the pH of a sodium
Q61: 24 karat gold contains _% gold.<br>A)24<br>B)25<br>C)5.0 x
Q64: A solution is prepared by dissolving calcium
Q108: In the Haber process, ammonia is synthesized
Q125: The mole fraction of urea (MW =
Q130: Which of the following liquids will have