Given the thermodynamic data in the table below, calculate the equilibrium constant (at 298 K) for the reaction: 2SO2 (g) + O2 (g) 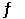 2SO3 (g)
2SO3 (g) 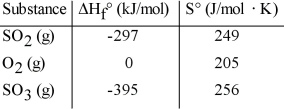
Definitions:
NPV Outcomes
The range of potential net present values resulting from differing scenarios in capital budgeting to evaluate investment project profitability.
Probability Distribution
A mathematical function that provides the probabilities of occurrence of different possible outcomes for an experiment.
NPV
The calculation of the current value of all future cash flows generated by a project, after accounting for the initial capital expenditure.
IRR
Internal Rate of Return; a metric used in capital budgeting to estimate the profitability of potential investments.
Q11: A sample of air from a home
Q23: The standard reduction potential, E°<sub>red</sub>, is proportional
Q25: The danger from mixing ammonia with bleach
Q76: Given the following reaction at equilibrium, if
Q81: Sulfur compounds in the atmosphere are equally
Q83: What is emitted in the nuclear transmutation,
Q108: The C-O bond dissociation energy in CO<sub>2</sub>
Q110: What current (in A)is required to plate
Q125: The half-life of <sup>223</sup>Ra is 11.4 days.
Q159: The reaction between nitrogen dioxide and water