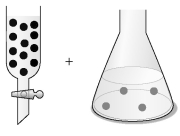
-The concentration of an aqueous solution of I3⁻ can be determined by a redox titration with aqueous sodium thiosulfate,Na2S2O3: 2 S2O32- (aq) + I3⁻ (aq) + → S4O62- (aq) + 3 I⁻ (aq)
Assume that the black spheres in the buret represent S2O32- ions,the gray spheres in the flask represent I3- ions,the concentration of the S2O32- ions in the buret is 0.120 M,and the volumes in the buret and the flask are identical.What is the concentration of the I3- in the flask,and what fraction of the S2O32- solution in the buret must be added to the flask to react with all the I3- ions?
Definitions:
Deductible
A deductible is an amount paid by an insured party before the insurance company covers the remaining costs of a claim.
Dependent
A person, often a child or elderly family member, whose livelihood and financial support are largely provided by someone else, typically a family member.
Doctor Bills
Invoices or financial charges for medical services rendered by a physician.
Deductible
The amount paid out of pocket by the policyholder before an insurance company pays a claim.
Q1: Molarity is defined as<br>A)moles of solute per
Q3: The electronegativity is 2.1 for H and
Q5: The first vibrational level for NaH lies
Q21: How many of the σ bonds in
Q39: An element that has the valence electron
Q60: The compound,Sn(IO<sub>3</sub>)<sub>2</sub>,is named<br>A)tin iodate(II).<br>B)tin(I)iodate.<br>C)tin(I)iodate(II).<br>D)tin(II)iodate.
Q92: What is the empirical formula for ethyl
Q122: Which element,indicated by letter on the periodic
Q166: Arrange the following spectral regions in order
Q193: In which pair are both formulas of