Assume that the conductivity of a solution depends only on the total concentration of dissolved ions and that you measure the conductivity of three different solutions while performing titrations in which
I.50.00 mL of 0.100 M aqueous CH3CO2H is titrated by addition of 0.100 M NaOH.
II.50.00 mL of 0.100 M aqueous NaBr is titrated by addition of 0.100 M AgNO3.
III.50.00 mL of 0.100 M aqueous CaCl2 is titrated by addition of 0.100 M Na2CO3. 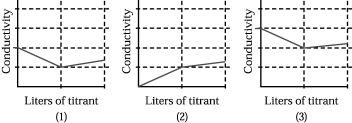
-Which of the above graphs corresponds to titration I?
Definitions:
Statutory Process
A formal procedure or series of steps established by statute (law) that organizations, governments, or individuals must follow.
Common Law
A legal system based on precedent and court rulings, as opposed to statutes or written legislation, forming a major part of the law in many English-speaking countries.
Statutory Assignment
The transfer of a legal right from one person to another pursuant to specific statutes.
Notice in Writing
A formal method of communication that is documented in a written format and often required by law or contracts to announce changes or intentions.
Q18: A hydrocarbon of unknown formula C<sub>x</sub>H<sub>y</sub> was
Q35: In the best Lewis structure for CO<sub>2</sub>,what
Q52: When melting S<sub>8</sub>,_ forces must be overcome
Q61: If the percent yield for the following
Q78: The chlorine atom in Cl<sub>2</sub> would be
Q83: What is the mass of 0.0500 mol
Q119: A student left 40.0 mL of a
Q129: If the volumes in the buret and
Q133: If the reaction of phosphate ion with
Q200: How many grams of KNO<sub>3</sub> are needed