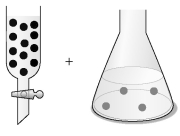
-The concentration of an aqueous solution of I3⁻ can be determined by a redox titration with aqueous sodium thiosulfate,Na2S2O3: 2 S2O32- (aq) + I3⁻ (aq) + → S4O62- (aq) + 3 I⁻ (aq)
Assume that the black spheres in the buret represent S2O32- ions,the gray spheres in the flask represent I3- ions,the concentration of the S2O32- ions in the buret is 0.120 M,and the volumes in the buret and the flask are identical.What is the concentration of the I3- in the flask,and what fraction of the S2O32- solution in the buret must be added to the flask to react with all the I3- ions?
Definitions:
Problems
Problems are obstacles or challenges that need to be resolved or overcome through the application of strategies and solutions.
Emotional Intelligence
The ability of individuals to recognize, understand, manage, and use their own emotions as well as the emotions of others effectively.
Prioritize
The action of arranging or dealing with tasks, activities, or goals according to their importance or urgency.
Allocating Time
The process of distributing or assigning time for specific activities or tasks.
Q10: For an electron in a given atom,the
Q35: In which of the following sets do
Q92: When K<sub>2</sub>SO<sub>4</sub>(aq)and Pb(NO<sub>3</sub>)<sub>2</sub>(aq)are mixed,a white colored precipitate
Q102: How many valence shell electrons does an
Q121: Of the following,which element has the highest
Q122: Which element,indicated by letter on the periodic
Q128: Rubidium belongs to the _ group of
Q131: An aqueous solution of H<sub> </sub>Cl is
Q142: Which of the following elements has chemical
Q223: Which is most likely to form a