Calculate the energy change in kJ/mol for the reaction 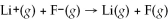 using the following information: Li(g) → Li+(g) + e- +520 kJ/mol
using the following information: Li(g) → Li+(g) + e- +520 kJ/mol
F(g) + e- → F-(g) -328 kJ/mol
Definitions:
Transcendence
The act of going beyond ordinary limits; in psychology, it refers to surpassing one's current self to achieve higher states of development or consciousness.
Individuation Process
The journey towards self-realization and wholeness, emphasizing the integration of the conscious and unconscious realms of the psyche.
Opposing Aspects
This term refers to contrasting elements or components within a system, theory, or phenomenon that exist in opposition to one another.
Individuation Process
A concept in psychology, particularly in analytical psychology, referring to the process of integrating different aspects of the consciousness and unconsciousness into a whole.
Q12: Which one of these spheres represents an
Q24: Wave (b)has the<br>A)higher amplitude and greater intensity
Q38: Which element has the most favorable (most
Q44: Which sphere most likely represents the Na⁺
Q77: List all the elements that have a
Q79: Which orbital hybridization is associated with a
Q85: A nitrogen atom in N<sub>2</sub> should have
Q87: What is the electron geometry and molecular
Q93: The highest note on a piano has
Q95: Which two ions have the same electron