Multiple Choice
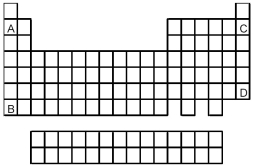
-Atoms of which element,indicated by letter on the periodic table above,would be expected to have the lowest first ionization energy,Ei1?
Definitions:
Related Questions
Q10: Atoms of which element,indicated by letter on
Q23: Under thermodynamic standard state conditions the element
Q27: The balanced equation for the gaseous state
Q38: Given that O<sub>2</sub> is paramagnetic and has
Q45: This reaction is likely to be<br>A)nonspontaneous at
Q59: How many lone pairs in the correct
Q82: Calculate the lattice energy for NaCl(s)using a
Q122: Which one of these spheres represents an
Q125: Calculate the energy change in kJ/mol for
Q185: In the drawing of acetaldehyde,CH<sub>3</sub>CHO,the largest partial