Calculate the total quantity of heat required to convert 50.0 g of liquid CCl4(l) from 25.0°C to gaseous CCl4 at 76.8°C (the normal boiling point for CCl4) ? The specific heat of CCl4(l) is 0.857 J/(g ∙ °C) ,its heat of fusion is 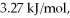 and its heat of vaporization is
and its heat of vaporization is 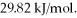
Definitions:
Environmentalist
An individual who advocates for or works towards protecting the natural environment from degradation and promoting sustainability.
Rio De Janeiro
A major city in Brazil, known for its Copacabana and Ipanema beaches, the 38m Christ the Redeemer statue atop Mount Corcovado, and its carnival festival.
Planting
The act of placing seeds or young plants in the ground to grow, which is fundamental for agriculture, gardening, and reforestation efforts.
Crops
Plants cultivated by humans on a large scale for consumption, animal feed, or other purposes.
Q16: Assign formal charges to each atom in
Q23: Which species does not have an octet
Q24: The intermediate,T,+ S is represented by<br>A)arrow C.<br>B)line
Q35: In the best Lewis structure for CO<sub>2</sub>,what
Q46: A gas occupies 22.4 L at STP
Q71: For the reaction I<sub>2</sub>(g)→ I<sub>2</sub>(s),ΔH° = -62.4
Q138: Calculate the total quantity of heat required
Q140: Iodine,I<sub>2</sub>(s),is more soluble in dichloromethane,CH<sub>2</sub>Cl<sub>2</sub>(l),than in water
Q151: Shown below is a model of SiH<sub>4</sub>
Q175: What are the F-Se-F bond angles in