Calculate the total quantity of heat required to convert 25.0 g of liquid CCl4(l) from 45.0°C to gaseous CCl4 76.8°C (the normal boiling point for CCl4) ? The specific heat of CCl4(l) is 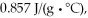 its heat of fusion is
its heat of fusion is 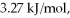 and its heat of vaporization is
and its heat of vaporization is 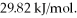
Definitions:
Concept Test
An evaluation or survey designed to gauge the potential market acceptance or appeal of a new product idea before its development.
New-product Idea
A concept or proposal for a product that has not been previously introduced to the market, often assessed during the product development phase.
Open Innovation
A business strategy that encourages the use of external and internal ideas and collaborations to drive innovation and speed up development processes.
Idea Generation
The process of creating, developing, and communicating new concepts or solutions.
Q10: Classify bonds in BeS as largely ionic,nonpolar
Q30: The heat of vaporization of water at
Q38: Which of the following compounds forms an
Q40: Which arrow represents ΔH<sub>soln</sub>?<br>A)arrow (a)<br>B)arrow (b)<br>C)arrow (c)<br>D)arrow
Q45: Which ionization process requires the most energy?<br>A)Pt(g)→
Q88: The electronegativity for both sulfur and carbon
Q92: Which element can expand its valence shell
Q160: In order to complete an electron-dot structure
Q162: When an electron is added to the
Q167: One mole of gas at 25°C in