Imagine a reaction that results in a change in both volume and temperature,as shown in the diagram below.What is the sign of the work being done and the sign of the enthalpy change involved in this reaction? 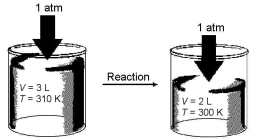
Definitions:
Dependable
Able to be trusted or relied upon; showing reliability and consistency in behavior or performance.
Team Player
A person who works well with others, often prioritizing group goals over personal achievements for the success of the team.
Motivated to Learn
Having a strong desire or willingness to gain knowledge or skills.
Peak Performance Success Formula
A strategy or set of principles designed to optimize efficiency and achieve the highest level of success in a particular field or activity.
Q19: Which period 2 element has successive first
Q22: Which drawing best shows the molecular polarity
Q26: A solution is prepared by dissolving 171
Q37: If the Earth's ozone (O<sub>3</sub>)layer has a
Q86: What is the F-B-F bond angle in
Q106: Which drawing best shows the direction of
Q115: In the reaction below,is energy released or
Q117: The octet rule is most likely to
Q125: An aqueous CsCl solution is 8.00 wt%
Q139: Based on the relative strengths of the