The plots below represent vapor pressure vs.temperature curves for diethyl ether,ethanol,mercury,and water,not necessarily in that order. 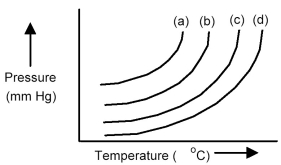
-Based on the relative strengths of the intermolecular forces of attraction of each substance,which is the most likely vapor pressure vs.temperature curve for water?
Definitions:
Supervised
Overseen or directed by an authority to ensure that tasks are carried out correctly or safely.
Wording
The choice and use of words and phrases in speech or writing.
Résumé
A formal, documented outline detailing an individual's academic history, professional experience, and qualifications for employment.
Mild Exaggerations
Slight overstatements or embellishments used to emphasize a point or make a story more interesting, without significantly distorting the truth.
Q11: A balloon filled with helium gas at
Q32: For the reaction shown below,what is the
Q32: A person is considered legally intoxicated with
Q35: The first-order reaction,2 N<sub>2</sub>O(g)→ 2 N<sub>2</sub>(g)+ O<sub>2</sub>(g),has
Q37: The reaction below is second order in
Q81: A lungful of air (500 mL)contains 4.1%
Q91: For a process at constant pressure,<br>A)ΔE =
Q104: Ethyl alcohol is produced by the fermentation
Q181: Methanol can be produced by the following
Q186: An aqueous solution has a normal boiling