Arrows in the energy diagram below represent enthalpy changes occurring in the exothermic formation of a solution:
ΔHsoln = enthalpy of solution
ΔHsolute-solute = enthalpy change involving solute-solute interactions
ΔHsolute-solvent = enthalpy change involving solute-solvent interactions
ΔHsolvent-solvent = enthalpy change involving solvent-solvent interactions 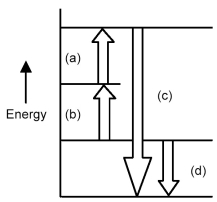
-Which arrow represents ΔHsolute-solvent?
Definitions:
Chemical Supply Company
A business that specializes in the distribution of chemicals for industrial, research, or consumer use.
Web Developer
A professional who specializes in the development of applications and solutions for the Internet, ranging from websites to services accessed via web browsers.
Hand Picker
An interface tool or device designed to simulate hand gestures or movements, often used in virtual reality or graphic design applications.
PCI DSS
The Payment Card Industry Data Security Standard, a set of security standards designed to ensure that all companies that accept, process, store, or transmit credit card information maintain a secure environment.
Q17: At 300 K a 500.0 mL solution
Q24: Identify the packing in the figure shown
Q34: For the reaction,N<sub> </sub>H<sub>3</sub>(g)→ N(g)+ 3 H(g),one
Q69: The secondary pollutants in photochemical smog are
Q76: Which type of bonding does sucrose form
Q100: What is the molality of a glucose
Q142: What is the rate law for this
Q158: The following set of data was obtained
Q175: A reaction has the rate law Rate
Q181: Methanol can be produced by the following