A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp = 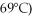 and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 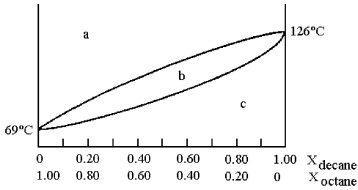
-Assume that you start with a mixture containing 0.80 mol of decane and 0.20 mol of octane,what region of the diagram corresponds to vapor?
Definitions:
Bankruptcy
A legal proceeding involving a person or business that is unable to repay their outstanding debts, leading to the distribution of assets to creditors.
Preferred Stock
A category of corporate ownership that holds a superior claim over the company's resources and profits compared to common stocks, typically offering consistent dividend payments.
Equity
The ownership interest of shareholders in a corporation, represented by their shares of stock.
Dividends
Payments made by a corporation to its shareholder members, often derived from profits.
Q29: The coordination number of each atom in
Q29: Given the reaction at a certain temperature:
Q49: A gaseous reaction occurs by a two-step
Q50: A reaction for which ΔE = -850
Q50: Nitric oxide reacts with oxygen to form
Q56: The equilibrium constant K<sub>c</sub><sup> </sup>for the reaction
Q79: What is the pressure (in mm Hg)of
Q115: A plot of 1/[BrO<sup>-</sup>] vs time is
Q123: A basketball is inflated to a pressure
Q138: Calculate the total quantity of heat required