The reaction for the decomposition of dinitrogen monoxide gas to form an oxygen radical is: 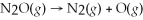 .If the activation energy is 250 kJ/mol and the frequency factor is 8.0 × 1011 s-1,what is the rate constant for the first-order reaction at 1000 K?
.If the activation energy is 250 kJ/mol and the frequency factor is 8.0 × 1011 s-1,what is the rate constant for the first-order reaction at 1000 K?
Definitions:
Multiple Voices
The presence of various distinct perspectives or narrative styles within a single text, conversation, or cognitive process.
Positive Symptoms
Manifestations of psychotic disorders such as delusions and hallucinations that represent an excess or distortion of normal functions.
Negative Symptoms
Features associated with reductions or deficits in normal emotional and behavioral functions, often seen in schizophrenia, such as apathy and lack of speech.
Social Withdrawal
The act of removing oneself from social interactions and activities, often as a response to anxiety, depression, or other stressors.
Q5: Which change in the system will drive
Q8: How many atoms are in one face-centered
Q22: A reaction has a rate constant,k =
Q67: In most liquid solutions,the component present in
Q79: A certain mineral crystallizes in the cubic
Q100: What is the molality of a glucose
Q147: Shown below is a concentration vs.time plot
Q152: Molecular hydrogen can be made from methane
Q162: When the concentration of A is doubled,the
Q182: What is the strongest acid among the