The reaction for the decomposition of dinitrogen monoxide gas to form oxygen radicals is: 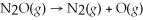 .If the rate constant is 3.04 × 10-2 s-1 and the frequency factor is 8.00 × 1011 s-1,what is the activation energy for the first-order reaction at 700°C?
.If the rate constant is 3.04 × 10-2 s-1 and the frequency factor is 8.00 × 1011 s-1,what is the activation energy for the first-order reaction at 700°C?
Definitions:
Fair Value Method
An accounting approach that assesses the price of an asset or liability based on current market conditions, rather than historical cost.
Goodwill
An intangible asset that arises when a company acquires another company for a price greater than the fair value of its net identifiable assets.
Measuring NCI
Stands for the process of valuing a Non-Controlling Interest, which represents ownership in a subsidiary not held by the parent company.
Ordinary Shares
Equity securities representing ownership in a company, which entitle holders to vote at shareholder meetings and receive dividends.
Q1: "Instantaneous rate" is defined as the<br>A)change in
Q13: A 0.700 g sample containing Ag<sub>2</sub>O and
Q22: The highest coordination number for spherical packing
Q35: From the following chemical reactions determine the
Q40: Which arrow represents ΔH<sub>soln</sub>?<br>A)arrow (a)<br>B)arrow (b)<br>C)arrow (c)<br>D)arrow
Q57: An acidic solution at 25°C will have
Q61: At 20°C and 0.28 atm pressure Xenon
Q61: What is the strongest monoprotic acid of
Q145: Given the hypothetical reaction: 2 A(s)+ x
Q176: Which one of the following species acts