The reaction A2 + B2 ⇌ 2 AB has an equilibrium constant Kc = 1.8.The following pictures represent reaction mixtures that contain A2 molecules (shaded) and B2 molecules (unshaded) ,and AB molecules.Which reaction mixture is at equilibrium? 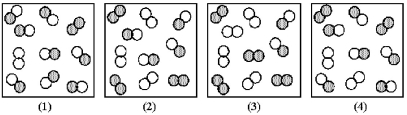
Definitions:
Socialist System of Economy
An economic system where the means of production are owned and controlled by the state or public, aiming for equal distribution of wealth and resources.
Q27: Which ion-dipole interaction results in the larger
Q34: Which of the following forms a molecular
Q63: The equilibrium constant,K<sub>p</sub>,equals 3.40 at 25°C for
Q69: Which ion-dipole interaction results in the larger
Q76: The Br∅nsted-Lowry acids in the chemical equation
Q76: What is the order of reaction with
Q83: A solution of 0.2113 g of water
Q97: A solution is prepared by dissolving 17.75
Q121: The reaction that occurs in a Breathalyzer,a
Q147: Shown below is a concentration vs.time plot