Calculate the pH for an aqueous solution of pyridine that contains 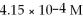 hydroxide ion.
hydroxide ion.
Definitions:
Carbon Dioxide
An invisible, scentless gas that results from the combustion of carbon and organic materials, as well as from the process of respiration, utilized by plants in photosynthesis.
Complete Combustion
A chemical reaction where a fuel is entirely burned with oxygen, producing a limited number of products like carbon dioxide and water, with maximum energy output.
Elemental Order
The organization of chemical elements based on their properties and behaviors, often represented in the periodic table.
Balanced Equation
An equation in chemistry that shows the same number of atoms of each element in the reactants and products, maintaining mass conservation.
Q4: Entropy is a measure of<br>A)free energy.<br>B)the heat
Q43: When equal molar amounts of the following
Q57: Which has the lowest entropy?<br>A)CH<sub>3</sub>OH(s,-26°C)<br>B)CH<sub>3</sub>OH(s,-13°C)<br>C)CH<sub>3</sub>OH(l,16°C)<br>D)CH<sub>3</sub>OH(l,30°C)
Q66: If K<sub>c</sub> is the equilibrium constant for
Q93: Hydroquinone,HOC<sub>6</sub>H<sub>6</sub>OH,can be formed by the reaction with
Q127: What is the relationship between K<sub>a</sub> and
Q130: The decomposition of hydrogen peroxide is given
Q153: The second-order reaction,2 Mn(CO)<sub>5</sub> → Mn<sub>2</sub>(CO)<sub>10</sub> has
Q170: In a galvanic cell,the half-reaction H<sub>2</sub>(g)+ 2
Q222: What is the pH of a solution