What is the hydronium ion concentration of a 0.200 M hypochlorous acid solution with 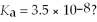 The equation for the dissociation of hypochlorous acid is: HOCl(aq) + H2O(l) ⇌ H3O+(aq) + OCl-(aq) .
The equation for the dissociation of hypochlorous acid is: HOCl(aq) + H2O(l) ⇌ H3O+(aq) + OCl-(aq) .
Definitions:
BPI Phase
Business Process Improvement Phase, a step in the process of analyzing and improving existing business processes to optimize performance and meet best practice standards.
"To Be" Process
A business blueprint detailing the desired optimized process or outcome that a company aims to achieve.
Root Causes
Fundamental reasons for the occurrence of a problem, which, when identified, allow for the creation of solutions to prevent recurrence.
BPI Team
A Business Process Improvement (BPI) Team focuses on analyzing business processes and workflows to identify inefficiencies and propose improvements to enhance productivity.
Q1: The reaction 2 H<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 H<sub>2</sub>O(g)is
Q11: If additional SCN<sup>-</sup> is added to the
Q47: In aqueous solution,hypobromite ion,BrO<sup>-</sup>,reacts to produce bromate
Q50: The following pictures represent three equilibrium mixtures
Q69: For the reaction N<sub>2</sub>(g)+ O<sub>2</sub>(g)→ 2 NO(g),ΔH°
Q107: How will the spontaneity of this reaction
Q114: Calculate the pH for an aqueous solution
Q121: At a certain temperature,hydrogen and iodine react
Q182: Which statement below is not true?<br>A)The cell
Q223: Bromothymol blue indicator changes color from yellow