Multiple Choice
SO3 reacts with H2O to form H2SO4.Which picture below correctly represents the curved arrow notation for the initial Lewis acid-Lewis base interaction in this reaction;what is the Lewis acid and the Lewis base? 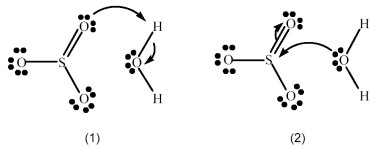
Definitions:
Related Questions
Q28: What is the percent dissociation of a
Q36: The balanced equation for the solubility equilibrium
Q55: Addition of 0.0125 mol HCl to 150
Q59: The decomposition of cyclopropane,was observed at 500°C
Q113: Which nonequilibrium mixtures will react in the
Q127: What is the relationship between K<sub>a</sub> and
Q130: At a certain temperature the equilibrium constant,K<sub>c</sub>,equals
Q131: The reaction below is second order in
Q151: Which of the above pictures represents a
Q198: What is the pH of a solution