Which metal sulfides can be precipitated from a solution that is 0.01 M in Mn2+,Zn2+,Pb2+ and Cu2+ and 0.10 M in H2S at a pH of 1.0? 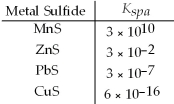
Definitions:
Days' Sales in Receivables
A financial ratio that measures how well a company is managing its accounts receivable by calculating the average number of days it takes to collect payments from customers.
Fiscal Year
A one-year period used for financial reporting and budgeting by businesses and governments, which may not align with the calendar year.
Dishonored Note
This term refers to a promissory note that has not been paid at maturity by the maker, resulting in default.
Interest for Days
Calculated as the interest rate on a loan or investment applied over a specified number of days.
Q3: What is the scandium ion concentration for
Q6: In the aquation reaction Co<sup>2+</sup> + 6
Q12: If an equal number of moles of
Q26: Chemical and physical changes can be classified
Q27: What is the strongest base among the
Q50: What is the characteristic pH-titrant curve for
Q66: If ΔG° is positive for a reaction,<br>A)K
Q67: A proton hydrated by ten water molecules
Q110: Which solution has the highest pH?<br>A)(1)<br>B)(2)<br>C)(3)<br>D)(4)
Q172: According to the balanced chemical equation 5