The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 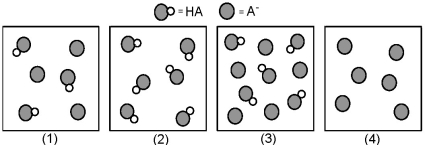
-Which solution has the highest pH?
Definitions:
Cheques
Written, dated, and signed instruments that direct a bank to pay a specific sum of money to the bearer or a specific individual.
Collection Time
The typical duration required for a company to collect payments due from its clients.
Amounts
Amounts refer to the quantities or sums of money involved in financial transactions or measurements.
Net Present Value
A calculation that compares the value of money now with the value of that money in the future, accounting for inflation and returns.
Q12: What is the reduction half reaction for
Q55: Addition of 0.0125 mol HCl to 150
Q57: An acidic solution at 25°C will have
Q96: What are the coefficients in front of
Q112: Which picture represents the system beyond the
Q129: Cyclohexane (C<sub>6</sub>H<sub>12</sub>)undergoes a molecular rearrangement in the
Q132: At 1000 K,K<sub>p</sub> = 19.9 for the
Q132: If solution (1)is a saturated solution of
Q181: The initial concentrations of Ag<sup>+</sup>(aq)and Cu<sup>2+</sup>(aq)are both
Q193: The equilibrium constant for the reaction below