The following plot shows two titration curves,each representing the titration of 50.00 mL of 0.100 M acid with 0.100 M NaOH. 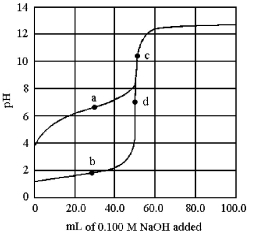
-Which points a-d represent the half-equivalence point and the equivalence point,respectively,for the titration of a weak acid?
Definitions:
Evaluation Efforts
The systematic assessment of the processes and/or outcomes of a program or project, with the aim of learning from experiences and making improvements.
Measurement
The process of determining the size, quantity, or degree of something, typically through the use of instruments or standardized tests.
Transactional Leader
A leadership style that focuses on the exchanges or transactions between leaders and their followers, often involving rewards for performance and corrections for underperformance.
Transformational Leader
A leadership style characterized by the ability to inspire and motivate followers toward significant change and innovation.
Q3: A reaction has ΔG° = + 21.5
Q21: Based on the balanced chemical equation shown
Q22: If ΔG° is negative for a reaction,<br>A)K
Q25: Which of the following solutions has the
Q47: Which statement is true concerning the standard
Q75: What is the hydronium ion concentration of
Q149: Predict the sign of ΔS for each
Q153: What is the molar solubility of AgCl
Q172: Which of the following metal hydroxides are
Q174: Which of the following titrations result in