The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 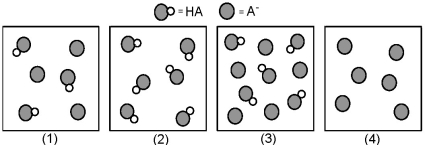
-Which of the solutions are buffer solutions?
Definitions:
Bureaucratic Control
A system of managing an organization through a formalized set of rules and procedures, ensuring standardization and consistency across operations.
Customer Feedback
The information, insights, and input that a business receives from individuals who use its products or services, which can be used for improvement and growth.
Sales And Profitability
measures of the effectiveness of a company's operations; where sales refer to the revenue from goods or services sold, and profitability is the extent to which this revenue exceeds the costs incurred.
Historical Approach
A method of research that examines past events and developments to understand the present and anticipate future trends.
Q18: A cell based on the reaction below
Q23: For a process to be at equilibrium,it
Q29: Human tears have a concentration of H<sub>3</sub>O<sup>+</sup>
Q41: An equilibrium mixture of CO,O<sub>2</sub> and CO<sub>2</sub>
Q67: If this reaction is exothermic,which picture (2)-(4)represents
Q75: Which of these solutions are buffers?<br>A)(1)and (2)<br>B)(1)and
Q98: Addition of 0.0125 mol KOH to 150
Q115: What is the pH of a solution
Q149: What is the strongest Br∅nsted-Lowry acid in
Q192: Identify the Br∅nsted-Lowry acid/base conjugate pairs.<br>A)(1)/(2)and (3)/(4)<br>B)(1)/(3)and