The following pictures represent solutions of AgCl,which may also contain ions other than Ag+ and Cl- which are not shown.Gray spheres represent Ag+ ions and dotted spheres represent Cl- ions. 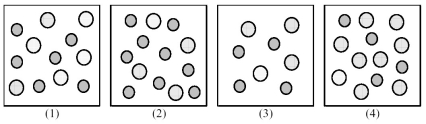
-If solution (1) is a saturated solution of AgCl,which of solutions (1) -(4) represents the solution after a small amount of AgNO3 is added and equilibrium is restored?
Definitions:
Q2: For the hypothetical reaction A + B<sup>x</sup>
Q16: What percentage of a radioactive substance remains
Q39: Write the equilibrium equation for the forward
Q48: The entropy change associated with the expansion
Q74: What is k in Boltzmann's formula,S =
Q97: Based on the balanced chemical equation shown
Q98: Redox reactions occurring in acid are evident
Q142: What is the approximate value of the
Q159: Find the equilibrium constant for the reaction:
Q196: The chlor-alkali industry is based on the