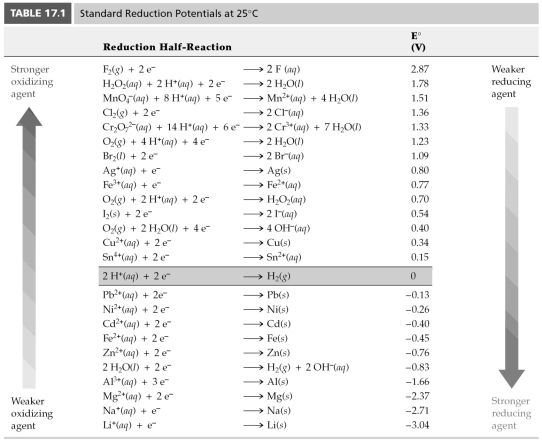
-Based on the following information, Cl2(g) + 2 e- → 2 Cl-(aq) E° = 1.36 V
Zn2+(aq) + 2 e- → 2 Zn(s) E° = -0.76 V
Which of the following chemical species is the strongest reducing agent?
Definitions:
Adjusting Journal Entry
Entries made in accounting records to update the balances of accounts before producing financial statements, ensuring they adhere to the accrual basis of accounting.
Salaries
Compensation given to employees for their labor or services, typically paid on a monthly or biweekly basis.
Five-day Week
A common work schedule that consists of five working days, usually Monday through Friday, followed by a two-day weekend.
Depreciation
The systematic allocation of the cost of a tangible asset over its useful life, recognizing the decrease in the value of the asset over time.
Q5: Which has the highest melting point?<br>A)La<br>B)W<br>C)Os<br>D)Hg
Q7: Calculate the cell potential E at 25°C
Q14: How does one commercially reduce the metal
Q17: Standard molar entropies,S°,in J/Kmol,are given below each
Q44: Precipitation of an ionic compound will occur
Q78: Which has the highest standard molar entropy
Q130: Which of the following buffer solutions will
Q140: Which of the above reaction mixtures has
Q149: The nuclear transformation potassium-40 argon-40 + ?
Q176: How many d electrons are there in