Consider the reaction CO(g) + 2H2(g) 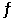 CH3OH(g)
CH3OH(g)
At room temperature,K is approximately 2 104,but at a higher temperature K is substantially smaller.Which of the following is true?
Definitions:
Federal Reserve Board
The governing body of the Federal Reserve System, responsible for overseeing the United States' central banking system and setting monetary policy.
Chairman
A person appointed or elected to preside over a meeting, board, or committee.
President
The head of state and head of government in a republic, typically elected to lead the country's executive branch and implement national laws and policies.
Board of Governors
The leading body of a central bank in many countries, responsible for overseeing the management and operations of the bank.
Q3: Why is the vapor pressure of cis-dibromoethene
Q5: Consider the following reaction at a certain
Q19: Which of the following statements is true?<br>A)A
Q36: Choose the effective pH range of a
Q56: Ceramic materials are hard,and tend not to
Q56: For the titration of 50.0 mL of
Q60: Calculate the solubility product of calcium hydroxide
Q72: Given: 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1039/.jpg" alt="Given: 2SO<sub>2</sub>(g)+
Q86: Why is gravel often used as a
Q155: What two types of bonds involving hydrogen