Multiple Choice
Consider the reaction 2HI(g) 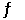 H2(g) + I2(g)
H2(g) + I2(g)
At 298 K,Kc = 1.3 103,whereas at 783 K,Kc = 2.2 102.Which of the following is true?
Definitions:
Related Questions
Q8: The boiling points of the Group 15
Q12: If an isolated system contained +5 kJ
Q18: Calculate the change in entropy of a
Q21: <font face="symbol"></font>U = 0 for the isothermal
Q26: What is the half-life of a second
Q30: Calculate the standard enthalpy of combustion of
Q34: A mixture consisting of 0.250 M N<sub>2</sub>(g)and
Q55: The following compounds are available as 0.10
Q57: For the proposed mechanism A + X
Q79: Consider the following cell: Ag(s)<font face="symbol"></font>Ag<sup>+</sup>(aq,0.100 M)mAg<sup>+</sup>(aq,0.100