Calculate the value of the equilibrium constant for the reaction AgCl(s) + 2NH3(aq) 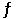 Ag(NH3) 2+(aq) + Cl(aq)
Ag(NH3) 2+(aq) + Cl(aq)
Given Ksp = 1.6 1010 for silver chloride and Kf = 1.6 107 for the ammonia complex of Ag+ ions,Ag(NH3) 2+.
Definitions:
Complex Sentence
A sentence that contains one independent clause and at least one dependent clause, expressing more nuanced ideas.
Sentence Structure
The arrangement of words and phrases to create well-formed sentences in a language, crucial for clarity and effectiveness in communication.
Subordinate Clauses
A part of a sentence that contains a subject and a verb but cannot stand alone as a complete sentence; it depends on the main clause to make sense.
Main Ideas
The central concepts or primary points that are being communicated in a text or speech.
Q12: The phase diagram for a pure substance
Q16: When the Ag(s)<font face="symbol"></font>AgCl(s)<font face="symbol"></font>Cl<font face="symbol"><sup></sup></font>(aq)electrode acts
Q17: What is the molarity of OH<font face="symbol"><sup></sup></font>
Q26: In the complex [Fe(CN)<sub>6</sub>]<sup>4</sup><font face="symbol"><sup></sup></font>,<br>A)iron is a
Q30: Consider the following compounds and their standard
Q40: Calculate the standard free energy of formation
Q52: The reaction 2NO(g)+ O<sub>2</sub>(g)<font face="symbol"></font> 2NO<sub>2</sub>(g)has <font
Q55: True or false: A cell that uses
Q70: If the standard enthalpy of combustion of
Q89: What is the conjugate base of H<sub>2</sub>PO<sub>4</sub><font