The value of ΔH° for the reaction below is +128.1 kJ: CH3OH (l) → CO (g) + 2H2 (g)
How many kJ of heat are consumed when 15.5 g of C 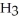 OH (l) decomposes as shown in the equation?
OH (l) decomposes as shown in the equation?
Definitions:
Accounts Receivable
Funds that clients or customers owe to a company for products or services that have been provided but not yet compensated for.
General Ledger
A comprehensive set of accounts that provides a complete record of all the financial transactions of a business.
Sales Tax
A consumption tax imposed by the government on sales of goods and services, collected by retailers at the point of sale and passed on to the government.
Cash Register
An electronic or mechanical device for registering and calculating transactions at a point of sale, often equipped with a cash drawer.
Q10: The value of ΔH° for the reaction
Q27: HNO<sub>2</sub> is a strong acid.
Q33: Which of the halogens has the highest
Q52: A typical fast food meal consists of
Q64: The formula for chromium (II)iodide is CrI<sub>2</sub>.
Q104: A tin atom has 50 electrons.Electrons in
Q113: Which of the subshells below do not
Q144: What is the concentration (M)of potassium ions
Q149: A great deal of oxygen is produced
Q162: The de Broglie wavelength of a 0.02900