The value of ΔH° for the reaction below is -6535 kJ. ________ kJ of heat are released in the combustion of 16.0 g of 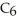
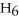 (l) ?
(l) ?
2C6H6 (l) + 15O2 (g) → 12CO2 (g) + 6H2O (l)
Definitions:
Examples
Specific instances or cases that are used to illustrate a concept, principle, or process.
Realistic Accuracy Model
The realistic accuracy model is a framework for understanding how accurate personality judgments are made, positing four key stages: relevance, availability, detection, and utilization of behavioral cues.
Displaying a Trait
The act of exhibiting or manifesting a particular characteristic or attribute that is part of one's personality or genetic makeup.
Q17: Which species below is the chromate ion?<br>A)Cr<sub>2</sub>O<sub>7</sub><sup>2-</sup><br>B)CrO<sub>4</sub><sup>2-</sup><br>C)CH<sub>3</sub>COO<sup>-</sup><br>D)CO<sub>3</sub><sup>2-</sup><br>E)None
Q51: Given the following reactions N<sub>2</sub> (g)+ O<sub>2</sub>
Q71: The elements in the _ period of
Q74: _ was the first _ gas to
Q79: 1 mole of which of the following
Q92: Calculate the work (kJ)done during a reaction
Q92: Sodium is much more apt to exist
Q137: Which quantum number determines the energy of
Q150: Water can be formed from the stoichiometric
Q163: What is the mass in grams of